Resources
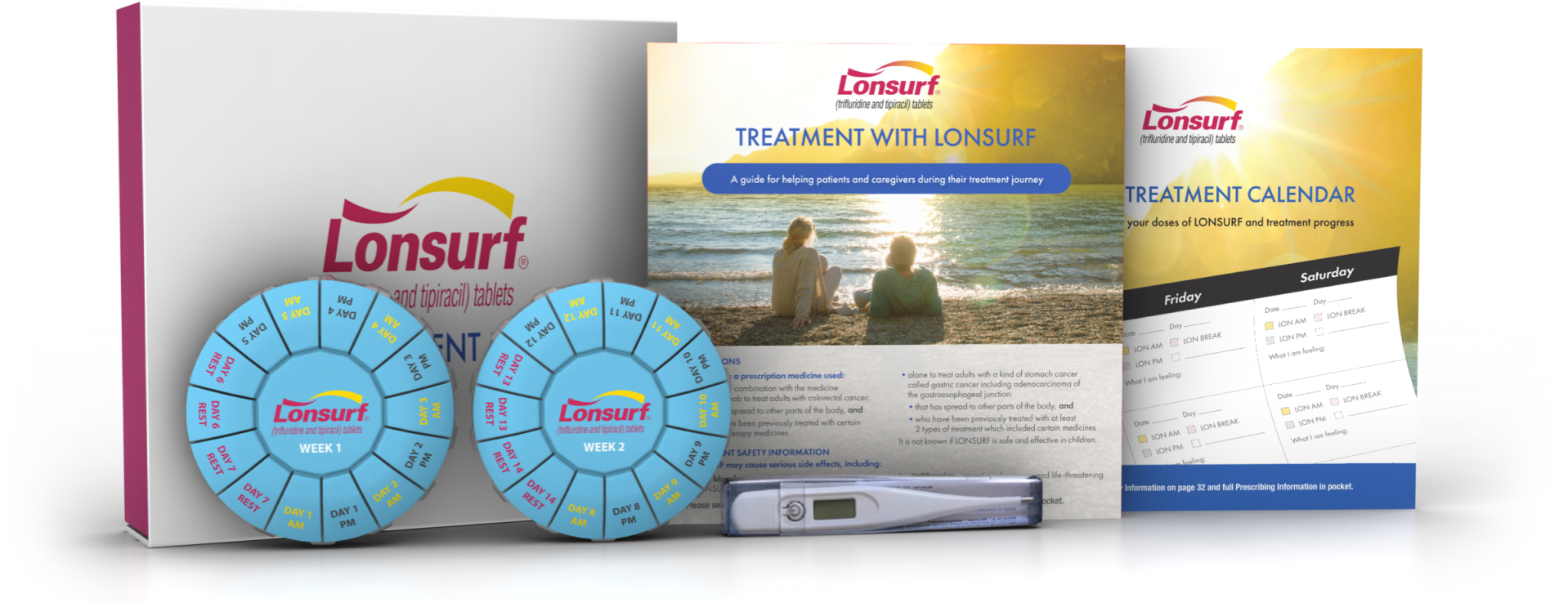
Patient Treatment Kit
The Patient Treatment Kit is meant to help you get the most out of your treatment. The materials provided are not meant to replace your healthcare provider's advice. Always speak with your healthcare provider about any questions or concerns you have. Together, you and your healthcare provider can develop a personalized treatment plan based on your needs.
The Patient Treatment Kit includes the following items:
- Patient & Caregiver Brochure
- Contains information on how LONSURF can help, how to take it, tips on managing common side effects, and services that may be able to help with the cost of LONSURF. You can also download this brochure below.
- LONSURF Treatment Calendar
- Helps you schedule and keep track of when to take your treatment, and note any side effects or important dates
- Pill Organizers
- For placement of LONSURF tablets to help you keep track of your treatment
- Thermometer
- Encourages you to check for fever while taking LONSURF to monitor for infections
or visit TaihoPatientSupport.com
- Doctor Discussion Guide
- Your doctor or nurse can help you find out whether LONSURF is right for you or your loved one. Be sure to bring this guide to your next appointment, and use the questions in it to help start the conversation.
- DOCTOR DISCUSSION GUIDE
- Vibrant, healthy recipes for cancer treatment and recovery
-
Discover nourishing, easy‑to‑follow recipes that you can make at home, all found in the NOURISH cookbook.
Inside, you will find an easy‑to‑use system of color‑coded symbols and meal prep icons designed to help you
manage some of the eating challenges associated with treatment.
Talk to your doctor before making any dietary changes. - COOKBOOK
- Foods to Avoid for People with Colon, Rectal, and Stomach Cancers
- This information is intended as a general guideline for patients undergoing treatment. Since you may experience unique symptoms depending on your disease and treatment, it's best to speak to your doctor for tailored recommendations. Depending on your sensitivities, some possible foods to avoid are:
-
- Caffeinated beverages (can lead to dehydration)
- High-fat foods
- Overly dry, spicy, salty, greasy, odorous, and acidic foods
- Gas-forming foods like beans, broccoli, cabbage, and onions
- High-lactose dairy
Downloadable Resources
Support Groups for Colon, Rectal, and Stomach Cancer
Get the support you need for metastatic colon, rectal, or stomach cancer
Meeting and learning from other people living with colon, rectal, or stomach cancer can be helpful. Connect with others online or in a local support group.
|
|
||
|---|---|---|
|
Colontown®
Visit colontown.org |
Colorectal Cancer Alliance
Visit ccalliance.org |
Fight Colorectal Cancer
Visit fightcolorectalcancer.org |
|
|
|||
|---|---|---|---|
|
Debbie's Dream Foundation
Visit debbiesdream.org |
Gastric Cancer Foundation Visit gastriccancer.org |
Hope for Stomach Cancer
Visit stocan.org |
No Stomach for Cancer
Visit nostomachforcancer.org |
|
|
|||
|---|---|---|---|
|
CancerCare®
Visit cancercare.org |
Cancer Support Community
Visit cancersupport |
GI Cancers Alliance
Visit gicancersalliance.org |
The Raymond Foundation
Visit theraymond |
Trademarks, registered or otherwise, are the property of their respective owners.
Frequently Asked Questions
Frequently Asked Questions about LONSURF
What is LONSURF?
LONSURF is an oral chemotherapy which contains two medicines in the same tablet:
- Trifluridine: May help prevent tumor growth by interfering with the tumor’s DNA
- Tipiracil: Helps trifluridine work longer
Is LONSURF chemotherapy?
Yes, it is a prescription chemotherapy tablet, taken orally.
How does LONSURF treat colorectal cancer or stomach cancer?
LONSURF includes two medicines in the same tablet:
- Trifluridine: May help prevent tumor growth by interfering with the tumor’s DNA
- Tipiracil: Helps trifluridine work longer
Why would my doctor prescribe LONSURF and bevacizumab together?
If you have advanced colon or rectal cancer, LONSURF may be used together with bevacizumab (also referred to by the brand name Avastin®), which may help slow or stop blood supply to the tumor. The use of LONSURF and bevacizumab together was approved in August 2023 based on the SUNLIGHT trial. In the trial, LONSURF with bevacizumab showed significant improvements in Overall Survival (10.8 vs. 7.5 months) and Progression-Free Survival (5.6 vs. 2.4 months).
What are the most common side effects associated with LONSURF when used alone or in combination with bevacizumab
The most common side effects of LONSURF when used alone include:
- Low blood counts, tiredness and weakness, nausea, decreased appetite, diarrhea, vomiting, stomach-area (abdominal) pain, and fever
The most common side effects of LONSURF when used in combination with bevacizumab include:
- Low blood counts, tiredness and weakness, nausea, certain abnormal liver function blood tests, decreased salt (sodium) in your blood, diarrhea, stomach-area (abdominal) pain, and decreased appetite
These are not all of the possible side effects of LONSURF. For more information, ask your healthcare provider. Call your healthcare provider for medical advice about side effects.
Please see full Prescribing Information.
How is LONSURF taken?
LONSURF is taken twice a day with food - the type of food does not matter. Do not retake doses of LONSURF that are vomited or missed and continue with the next scheduled dose.
LONSURF comes in 2 strengths: 15mg and 20mg tablets.* Your healthcare provider may prescribe both strengths for your prescribed dose.
What is the LONSURF Dosing Schedule?
LONSURF dosing schedule:
- You will take LONSURF twice a day (with morning and evening meals) for 5 days, and then rest for the next 2 days. This goes on for 2 weeks. Then you will not take LONSURF for 2 weeks (14 days). This completes the 28-day LONSURF treatment cycle.
- This is repeated for as long as your healthcare provider says. Always follow all of your healthcare provider’s directions carefully.
Create a personalized treatment calendar at LONSURF.com/mycalendar to help you schedule and keep track of your treatment. Your healthcare provider may give you a treatment calendar as well.
How is bevacizumab administered when prescribed with LONSURF?
For most patients with colorectal cancer, LONSURF may be prescribed to be used with bevacizumab. Bevacizumab is given as an infusion once every 2 weeks (on Days 1 and 15 of your LONSURF treatment cycle) by a doctor or nurse.
What should I do if I vomit after taking LONSURF?
Do not retake doses of LONSURF that are vomited or missed and continue with the next scheduled dose. Tell your healthcare provider if you have nausea or vomiting that gets worse or does not go away.
How should LONSURF be stored and handled?
Store LONSURF at room temperature between 68°F to 77°F (20°C to 25°C). Do not store LONSURF with other medicines. Keep LONSURF in its own container. If you store your tablets outside of the original container, any unused LONSURF tablets should be disposed of after 30 days.
Wash your hands after handling LONSURF. Even though it is a pill, it is still chemotherapy. Make sure your caregiver wears gloves when handling LONSURF. Note that there is a packet inside the bottle that helps absorb moisture. Do not swallow this material. Keep LONSURF out of the reach of children.
What if my loved one or I have a question about LONSURF, and the doctor's office is not available?
To get answers to your questions about LONSURF, contact us at 1‑844‑TAIHO‑4U (1‑844‑824‑4648).
Does Taiho Oncology offer any financial assistance programs?
Yes. Taiho Oncology Patient Support is here to help you obtain access to your Taiho Oncology Medicine. Our program offers a benefits review to confirm coverage, co-pay assistance enrollment for eligible patients to receive a LONSURF copay card which may reduce co-pay responsibility to $0, specialty pharmacy prescription coordination, and personalized nurse support for patients taking LONSURF.
For more information about LONSURF financial support, call 1‑844‑TAIHO‑4U (1‑844‑824‑4648) or go to TaihoPatientSupport.com.
Why would my doctor change my LONSURF treatment plan?
Your doctor may change your LONSURF treatment plan for various reasons including:
- adjusting your dosage due to side effects you may be experiencing such as a decrease in white blood cells, red blood cells, or platelets, or if you experience other serious side effects.
- adding bevacizumab (also known as Avastin) to be used in combination with LONSURF which the SUNLIGHT clinical trial showed meaningfully improvements in treatment outcomes.
Talk to your healthcare provider about any changes to your treatment plan.
How could my doctor change my LONSURF treatment plan?
There are 3 ways your doctor may change your treatment plan. It is not uncommon to receive a change in your treatment plan. In each case, your doctor may first delay your treatment. Delaying your treatment is an important step your doctor may take before deciding whether to:
- Resume your LONSURF treatment at the same dose
- Resume your LONSURF treatment at a lower dose
- Stop your LONSURF treatment permanently
How could low white blood cell counts affect me and my LONSURF treatment?
Low white blood cell counts can make you more likely to get serious infections. Serious infections could be fatal. Your doctor may change your treatment to prevent blood cell counts from lowering to a dangerous level.
Tell your doctor right away if you develop any signs of infection such as fever, chills, or body aches. You can use the thermometer in the Treatment Kit to check your temperature each day.
Does LONSURF cause hair loss?
Every patient can react differently to therapy. However, when LONSURF was used as a single agent, 7% of patients experienced some sort of alopecia (also known as hair loss) compared to 1% of patients receiving placebo.
What is metastatic colon or rectal cancer?
Colon and rectal cancer are often referred to as colorectal cancer. It includes cancer that starts in the colon or rectum, which are both parts of the lower digestive system. Metastatic means that the cancer has spread to other parts of the body. Together, colon and rectal cancer are the fourth most common in the United States among men and women.
What is metastatic stomach or gastroesophageal junction cancer?
Stomach cancer is often referred to as gastric cancer. It includes cancer that starts to grow in the lining of the stomach. A similar type of cancer is gastroesophageal junction cancer (also known as GE Junction or GEJ). This is cancer that starts to grow in the place where the esophagus and the stomach meet. Metastatic means that the cancer has spread to other parts of the body. While it is less common in the United States, stomach cancer is the fifth most common cancer in the world.
INDICATIONS
INDICATIONS
LONSURF is a prescription medicine used:
- alone or in combination with the medicine bevacizumab to treat adults with colorectal cancer:
- that has spread to other parts of the body, and
- who have been previously treated with certain chemotherapy medicines
- alone to treat adults with a kind of stomach cancer called gastric cancer including adenocarcinoma of the gastroesophageal junction:
- that has spread to other parts of the body, and
- who have been previously treated with at least 2 types of treatment which included certain medicines
It is not known if LONSURF is safe and effective in children.


INDICATIONS
LONSURF is a prescription medicine used:
- alone or in combination with the medicine bevacizumab to treat adults with colorectal cancer:
- that has spread to other parts of the body, and
- who have been previously treated with certain chemotherapy medicines
- alone to treat adults with a kind of stomach cancer called gastric cancer including adenocarcinoma of the gastroesophageal junction:
- that has spread to other parts of the body, and
- who have been previously treated with at least 2 types of treatment which included certain medicines
It is not known if LONSURF is safe and effective in children.
IMPORTANT SAFETY INFORMATION
LONSURF may cause serious side effects, including:
- Low blood counts. Low blood counts are common with LONSURF and can sometimes be severe and life-threatening. LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts. Your healthcare provider may lower your dose of LONSURF or stop LONSURF if you have low white blood cell or platelet counts
Tell your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with LONSURF: fever, chills, or body aches.
Before taking LONSURF, tell your healthcare provider about all of your medical conditions, including if you:
- Have kidney or liver problems
- Are pregnant or plan to become pregnant. LONSURF can harm your unborn baby
- Females who can become pregnant: Your healthcare provider will do a pregnancy test before you start treatment with LONSURF. You should use effective birth control during and 6 months after the last dose of treatment with LONSURF. Tell your healthcare provider immediately if you become pregnant
- Males, while on treatment and for 3 months after your last dose of LONSURF, you should use a condom during sex with female partners who are able to become pregnant. Tell your healthcare provider right away if your partner becomes pregnant while you are taking LONSURF
- Are breastfeeding or plan to breastfeed. It is not known if LONSURF passes into your breast milk. Do not breastfeed during treatment with LONSURF and for 1 day after your last dose of LONSURF
Tell your healthcare provider about all the prescription and over-the-counter medicines, vitamins, and herbal supplements you take.
The most common side effects of LONSURF when used alone include low blood counts, tiredness and weakness, nausea, decreased appetite, diarrhea, vomiting, stomach-area (abdominal) pain, and fever.
The most common side effects of LONSURF when used in combination with bevacizumab include low blood counts, tiredness and weakness, nausea, certain abnormal liver function blood tests, decreased salt (sodium) in your blood, diarrhea, stomach-area (abdominal) pain, and decreased appetite.
Tell your healthcare provider if you have nausea, vomiting, or diarrhea that is severe or that does not go away.
These are not all of the possible side effects of LONSURF. For more information, ask your healthcare provider. Call your doctor for medical advice about side effects.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.



